 |
| (image taken from Clean Body Living website) |
We often notice this sign saying ‘BPA free’ when we walk down the aisles of hypermarket displaying various types of plastic drinking bottles. It seems like a must for the prestigious plastic bottle manufacturers today to put the sign on their products so that customers will be like ‘oh ok, BPA must be something nasty, since it says it is free from this substance, it must be safe to use’, without actually knowing what BPA is.
Back to 1891…
BPA stands for bisphenol A, a chemical substances that is used in manufacturing ofpolycarbonate plastic (PC) derived from petroleum. It was first synthesized as early as 1891 by a Russian chemist named Aleksandr Dianin (but it was first mentioned in scientific paper in 1905 by Thomas Zincke from Germany, and of course it was written in German that I couldn’t understand the content). Only after over 60 years, in 1953, two scientists – Dr. Hermann Schnell and Dr. Daniel Fox – respectively discovered PC through the reaction between BPA and phosgene. Both of them were amazed by its durability and strength, and continued developing this polymer. At the beginning, this material was used in electrical and electronic appliances and then slowly moved into industries producing plastic bottle and lining of canned food.
So it was ‘safe’ to be used at that time?
During early 1960’s, United States Food and Drug Administration (FDA) approved the usage of PC in food packaging. In 2008, they released a statement saying that BPA was safe to be used in food packaging without posing an acute health risk to the general population until 2013,amendment on food additive regulations was made that the usage of BPA in baby bottles, sippy cups and baby formula packaging is no longer authorised. However, it is still considered as safe to use BPA in other normal food packaging in United States.
Actually in 1930’s, British chemist, Charles Edwards Dodds had his major scientific discovery in synthetic artificial oestrogen and he already recognised BPA as one of them. However, there was no evidence about its threat towards human’s health so it didn’t receive much public’s attention.
 |
| You might want to look at it the chemistry way. Estradiol is one of the estrogens produced in human body. (image taken from My Wilde World blog) |
How dangerous is BPA?
BPA is considered as one of the endocrine disruptors which obstructs the normal functioning of hormones in human body by mimicking estrogens, a type of female sex hormones. They interact with the estrogen receptors in human body, causing our body to misunderstand that we actually produces those estrogens, leading to various physiological effects and health problems. If the concentration of BPA is very high, it will inhibit the function of androgens, which is the male hormones (I believe you can imagine the consequences).
One study shows that BPA can be metabolised by human body and be eliminated through urine (therefore urine test is carried out to measure the exposure rate). So low level of BPA might not bring harm to human body as long as the metabolism rate of your body is good, this makes newborn babies more susceptible towards the impact from BPA, but not foetus in mother’s womb because mother will do the metabolism work before transferring the blood to the foetus. However, according to the Society for Endocrinology, there are a few journals relating BPA to cancers such as breast cancer and thyroid tumour.
Maybe before we know what is the negative effects brought by BPA to human body, we should look at how we are exposed to this chemical. Basically our exposure to BPA is though ingestion, where the chemical leaches from the container or packaging into the food we consume. A few laboratory studies have been done to investigate whether BPA will leach from the PC plastic under normal use or other conditions. It shows that high temperature, acidic or alkaline environment increase the rate of polymer degradation, resulting in the leaching of BPA with higher rate, which is quite logical, that’s why we don’t usually use plastic container for hot food. However, when the PC plastic container was simulated normal use (rinsing, washing, storing food simulating solvent, room temperature etc.), BPA was still found leaching from the plastic.
How about cold food? Maybe if we store only cold stuff using PC plastic, the leaching can be prevented? Well, a study was done in Harvard College where 77 students were asked to avoid using PC plastic container for food and drink consumption in one week, and then take cold bevarage from PC plastic bottle for another week. Their urine was collected during the end of the first week and the end of the second week for BPA analysis. The results showed that the urinary BPA concentration had risen for 69%! This certainly surprised me that this light-weighted and heat-resistant plastic is actually not as stable as claimed.
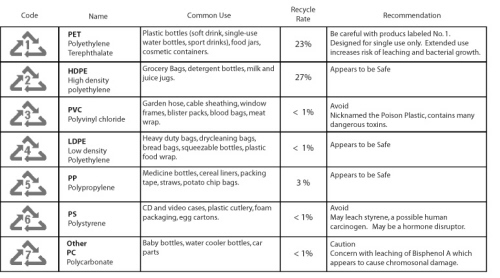 |
| (image taken from Plastic Free Bottle website) |
How do we know whether the plastic containers contain BPA?
It is clearly stated in the image above (maybe slightly blurry) on the types of plastics and their main components. PC plastic falls under the code 7, so you may now check on the bottom of the plastic bottle you are using now and start to feel worry. As I said earlier in this article, there are many plastic bottles sold out there displaying the proud sign of ‘BPA-free’. That doesn’t mean that ‘hooray the bottle is safe to be used’ because the BPA component of the bottle might be replaced by other chemical with unknown toxicity. So…… To be safe, use high quality stainless steel (no acidic liquid please!) or glass as your regular water bottle, if you don’t mind them being heavy. They are certainly the safe options!
References:
Caliendo, H. (2012, June 28). History of BPA [Article]. Retrieved from Packaging Digest website: http://www.packagingdigest.com/
Hengstler, J. G., Foth, H., Gebel, T., Kramer, P. J., Lilienblum, W., Schweinfurth, H., Völkel, W., Wollin, K. M. & Gundert-Remy, U. (2011). Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Critical Reviews in Toxicology, 41 (4), 263 – 291.
Miller, G. T. & Spoolman, S. E. (2012). Environmental hazards and human health. In Living in the environment (pp. 427 – 454). Canada: Yolanda Cossio
Carwile, J. L., Luu, H. T., Bassett, L. S., Driscoll, D. A., Yuan, C., Chang, J. Y., . . . Michels, K. B. (2009). Polycarbonate bottle use and urinary bisphenol A concentrations. Environmental Health Perspectives, 117 (9), 1368 – 1372.
P/S: This article was taken from the Confessions of An Environmental Student blog.




